Petroleum
Petroleum (L. petroleum, from early 15c. "petroleum, rock oil" (mid-14c. in Anglo-French), from Medieval Latin petroleum, from Latin: petra: "rock" + oleum: "oil".[1][2][3]) is a naturally occurring, yellow-to-black liquid found in geological formations beneath the Earth's surface, which is commonly refined into various types of fuels.
It consists of hydrocarbons of various molecular weights and other organic compounds.[4] The name petroleum covers both naturally occurring unprocessed crude oil and petroleum products that are made up of refined crude oil. A fossil fuel, petroleum is formed when large quantities of dead organisms, usually zooplankton and algae, are buried underneath sedimentary rock and subjected to both intense heat and pressure.
Petroleum has mostly been recovered by oil drilling, (natural petroleum springs are rare). This comes after the studies of structural geology (at the reservoir scale), sedimentary basin analysis, reservoir characterization (mainly in terms of the porosity and permeability of geologic reservoir structures).[5][6] It is refined and separated, most easily by distillation, into a large number of consumer products, from gasoline (petrol) and kerosene to asphalt and chemical reagents used to make plastics and pharmaceuticals.[7] Petroleum is used in manufacturing a wide variety of materials,[8] and it is estimated that the world consumes about 90 million barrels each day.
Concern over the depletion of the earth's finite reserves of oil, and the effect this would have on a society dependent on it, is a concept known as peak oil. The use of fossil fuels, such as petroleum, has a negative impact on Earth's biosphere, damaging ecosystems through events such as oil spills and releasing a range of pollutants into the air including ground-level ozone and sulfur dioxidefrom sulfur impurities in fossil fuels. The burning of fossil fuels plays a contributory role in the current episode of global warming.
Contents
[hide]- 1Etymology
- 2History
- 3Composition
- 4Chemistry
- 5Empirical equations for thermal properties
- 6Formation
- 7Reservoirs
- 8Classification
- 9Petroleum industry
- 10Price
- 11Uses
- 12Petroleum by country
- 13Environmental effects
- 14Alternatives to petroleum
- 15Future of petroleum production
- 16See also
- 17Notes
- 18References
- 19Further reading
- 20External links
Etymology[edit]
The word petroleum comes from Greek: πέτρα (petra) for rocks and Greek: ἔλαιον (elaion) for oil. The term was found (in the spelling "petraoleum") in 10th-century Old English sources.[9] It was used in the treatise De Natura Fossilium, published in 1546 by the German mineralogist Georg Bauer, also known as Georgius Agricola.[10] In the 19th century, the term petroleum was often used to refer to mineral oils produced by distillation from mined organic solids such as cannel coal (and later oil shale), and refined oils produced from them; in the United Kingdom, storage (and later transport) of these oils were regulated by a series of Petroleum Acts, from the Petroleum Act 1863 onwards.
History[edit]
Main article: History of the petroleum industry
Early history[edit]
Petroleum, in one form or another, has been used since ancient times, and is now important across society, including in economy, politics and technology. The rise in importance was due to the invention of the internal combustion engine, the rise in commercial aviation, and the importance of petroleum to industrial organic chemistry, particularly the synthesis of plastics, fertilizers, solvents, adhesives and pesticides.
More than 4000 years ago, according to Herodotus and Diodorus Siculus, asphalt was used in the construction of the walls and towers of Babylon; there were oil pits near Ardericca (near Babylon), and a pitch spring on Zacynthus.[11]Great quantities of it were found on the banks of the river Issus, one of the tributaries of the Euphrates. Ancient Persian tablets indicate the medicinal and lighting uses of petroleum in the upper levels of their society. By 347 AD, oil was produced from bamboo-drilled wells in China.[12] Early British explorers to Myanmar documented a flourishing oil extraction industry based in Yenangyaung that, in 1795, had hundreds of hand-dug wells under production.[13] The mythological origins of the oil fields at Yenangyaung, and its hereditary monopoly control by 24 families, indicate very ancient origins.
Pechelbronn (Pitch fountain) is said to be the first European site, where petroleum has been explored and used. The still active Erdpechquelle, a spring where petroleum appears mixed with water has been used since 1498, e.g. for medical purposes. Oil sands have been mined since the 18th century. [14]
In Wietze in lower Saxony, natural asphalt/bitumen has been explored since the 18th century. [15] Both in Pechelbronn as in Wietze, the coal industry dominated the petroleum technologies.[16]
Modern history[edit]
The process to distill kerosene from petroleum was invented by a Polish chemist Filip Neriusz Walter.
Scottish chemist James Young noticed a natural petroleum seepage in the Riddings colliery at Alfreton, Derbyshire from which he distilled a light thin oil suitable for use as lamp oil, at the same time obtaining a thicker oil suitable for lubricating machinery. In 1848 Young set up a small business refining the crude oil.
Young eventually succeeded, by distilling cannel coal at a low heat, in creating a fluid resembling petroleum, which when treated in the same way as the seep oil gave similar products. Young found that by slow distillation he could obtain a number of useful liquids from it, one of which he named "paraffine oil" because at low temperatures it congealed into a substance resembling paraffin wax.[17]
The production of these oils and solid paraffin wax from coal formed the subject of his patent dated 17 October 1850. In 1850 Young & Meldrum and Edward William Binney entered into partnership under the title of E.W. Binney & Co. at Bathgate in West Lothian and E. Meldrum & Co. at Glasgow; their works at Bathgate were completed in 1851 and became the first truly commercial oil-works in the world with the first modern oil refinery, using oil extracted from locally mined torbanite, shale, and bituminous coal to manufacturenaphtha and lubricating oils; paraffin for fuel use and solid paraffin were not sold until 1856.[18]
The world's first oil refinery was built in 1856 by Ignacy Łukasiewicz.[19] His achievements also included the discovery of how to distill kerosene from seep oil, the invention of the modern kerosene lamp (1853), the introduction of the first modern street lamp in Europe (1853), and the construction of the world's first modern oil well (1854).[20]
The demand for petroleum as a fuel for lighting in North America and around the world quickly grew.[21] Edwin Drake's 1859 well near Titusville, Pennsylvania, is popularly considered the first modern well. Already 1858 Georg Christian Konrad Hunäus had found a significant amount petroleum while drilling for lignite 1858 in Wietze, Germany. Wietze later provided about 80% of the German consumption in the Wilhelminian Era.[22] The production stopped 1963, but Wietze has hosted a Petroleum Museum since 1970.[23]
Drake's well is probably singled out because it was drilled, not dug; because it used a steam engine; because there was a company associated with it; and because it touched off a major boom.[24] However, there was considerable activity before Drake in various parts of the world in the mid-19th century. A group directed by Major Alexeyev of the Bakinskii Corps of Mining Engineers hand-drilled a well in the Baku region in 1848.[25] There were engine-drilled wells in West Virginia in the same year as Drake's well.[26] An early commercial well was hand dug in Poland in 1853, and another in nearby Romania in 1857. At around the same time the world's first, small, oil refinery was opened at Jasło in Poland, with a larger one opened at Ploiești in Romania shortly after. Romania is the first country in the world to have had its annual crude oil output officially recorded in international statistics: 275 tonnes for 1857.[27][28]
The first commercial oil well in Canada became operational in 1858 at Oil Springs, Ontario (then Canada West).[29] Businessman James Miller Williams dug several wells between 1855 and 1858 before discovering a rich reserve of oil four metres below ground.[30]Williams extracted 1.5 million litres of crude oil by 1860, refining much of it into kerosene lamp oil.[29] Williams's well became commercially viable a year before Drake's Pennsylvania operation and could be argued to be the first commercial oil well in North America.[29] The discovery at Oil Springs touched off an oil boom which brought hundreds of speculators and workers to the area. Advances in drilling continued into 1862 when local driller Shaw reached a depth of 62 metres using the spring-pole drilling method.[31]On January 16, 1862, after an explosion of natural gas Canada's first oil gusher came into production, shooting into the air at a recorded rate of 3,000 barrels per day.[32] By the end of the 19th century the Russian Empire, particularly the Branobel company inAzerbaijan, had taken the lead in production.[33]
Access to oil was and still is a major factor in several military conflicts of the twentieth century, including World War II, during which oil facilities were a major strategic asset and were extensively bombed.[34] The German invasion of the Soviet Union included the goal to capture the Baku oilfields, as it would provide much needed oil-supplies for the German military which was suffering from blockades.[35] Oil exploration in North America during the early 20th century later led to the US becoming the leading producer by mid-century. As petroleum production in the US peaked during the 1960s, however, the United States was surpassed by Saudi Arabia and the Soviet Union.
Today, about 90 percent of vehicular fuel needs are met by oil. Petroleum also makes up 40 percent of total energy consumption in the United States, but is responsible for only 1 percent of electricity generation.[citation needed] Petroleum's worth as a portable, dense energy source powering the vast majority of vehicles and as the base of many industrial chemicals makes it one of the world's most important commodities. Viability of the oil commodity is controlled by several key parameters, number of vehicles in the world competing for fuel, quantity of oil exported to the world market (Export Land Model), Net Energy Gain (economically useful energy provided minus energy consumed), political stability of oil exporting nations and ability to defend oil supply lines.
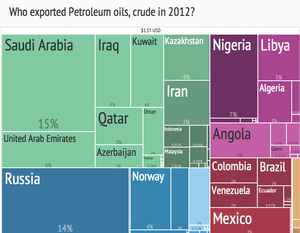
The top three oil producing countries are Russia, Saudi Arabia and the United States.[36] About 80 percent of the world's readily accessible reserves are located in the Middle East, with 62.5 percent coming from the Arab 5: Saudi Arabia, UAE, Iraq, Qatar and Kuwait. A large portion of the world's total oil exists as unconventional sources, such as bitumen in Canada and extra heavy oil in Venezuela. While significant volumes of oil are extracted from oil sands, particularly in Canada, logistical and technical hurdles remain, as oil extraction requires large amounts of heat and water, making its net energy content quite low relative to conventional crude oil. Thus, Canada's oil sands are not expected to provide more than a few million barrels per day in the foreseeable future.
Composition[edit]
In its strictest sense, petroleum includes only crude oil, but in common usage it includes all liquid, gaseous, and solid hydrocarbons. Under surface pressure and temperature conditions, lighter hydrocarbons methane, ethane, propane and butane occur as gases, while pentane and heavier ones are in the form of liquids or solids. However, in an underground oil reservoir the proportions of gas, liquid, and solid depend on subsurface conditions and on the phase diagram of the petroleum mixture.[37]
An oil well produces predominantly crude oil, with some natural gas dissolved in it. Because the pressure is lower at the surface than underground, some of the gas will come out of solution and be recovered (or burned) as associated gas or solution gas. A gas wellproduces predominantly natural gas. However, because the underground temperature and pressure are higher than at the surface, the gas may contain heavier hydrocarbons such as pentane, hexane, and heptane in the gaseous state. At surface conditions these will condense out of the gas to form natural gas condensate, often shortened to condensate. Condensate resembles gasoline in appearance and is similar in composition to some volatile light crude oils.
The proportion of light hydrocarbons in the petroleum mixture varies greatly among different oil fields, ranging from as much as 97 percent by weight in the lighter oils to as little as 50 percent in the heavier oils and bitumens.
The hydrocarbons in crude oil are mostly alkanes, cycloalkanes and various aromatic hydrocarbons while the other organic compounds contain nitrogen, oxygen and sulfur, and trace amounts of metals such as iron, nickel, copper and vanadium. Many oil reservoirs contain live bacteria.[38] The exact molecular composition varies widely from formation to formation but the proportion of chemical elements vary over fairly narrow limits as follows:[39]
| Element | Percent range |
|---|---|
| Carbon | 83 to 85% |
| Hydrogen | 10 to 14% |
| Nitrogen | 0.1 to 2% |
| Oxygen | 0.05 to 1.5% |
| Sulfur | 0.05 to 6.0% |
| Metals | < 0.1% |
Four different types of hydrocarbon molecules appear in crude oil. The relative percentage of each varies from oil to oil, determining the properties of each oil.[37]
| Hydrocarbon | Average | Range |
|---|---|---|
| Alkanes (paraffins) | 30% | 15 to 60% |
| Naphthenes | 49% | 30 to 60% |
| Aromatics | 15% | 3 to 30% |
| Asphaltics | 6% | remainder |
Crude oil varies greatly in appearance depending on its composition. It is usually black or dark brown (although it may be yellowish, reddish, or even greenish). In the reservoir it is usually found in association with natural gas, which being lighter forms a gas cap over the petroleum, and saline water which, being heavier than most forms of crude oil, generally sinks beneath it. Crude oil may also be found in semi-solid form mixed with sand and water, as in the Athabasca oil sands in Canada, where it is usually referred to as crudebitumen. In Canada, bitumen is considered a sticky, black, tar-like form of crude oil which is so thick and heavy that it must be heated or diluted before it will flow.[41] Venezuela also has large amounts of oil in the Orinoco oil sands, although the hydrocarbons trapped in them are more fluid than in Canada and are usually called extra heavy oil. These oil sands resources are called unconventional oil to distinguish them from oil which can be extracted using traditional oil well methods. Between them, Canada and Venezuela contain an estimated 3.6 trillion barrels (570×109 m3) of bitumen and extra-heavy oil, about twice the volume of the world's reserves of conventional oil.[42]
Petroleum is used mostly, by volume, for producing fuel oil and gasoline, both important "primary energy" sources. 84 percent by volume of the hydrocarbons present in petroleum is converted into energy-rich fuels (petroleum-based fuels), including gasoline, diesel, jet, heating, and other fuel oils, and liquefied petroleum gas.[43] The lighter grades of crude oil produce the best yields of these products, but as the world's reserves of light and medium oil are depleted, oil refineries are increasingly having to process heavy oil and bitumen, and use more complex and expensive methods to produce the products required. Because heavier crude oils have too much carbon and not enough hydrogen, these processes generally involve removing carbon from or adding hydrogen to the molecules, and using fluid catalytic cracking to convert the longer, more complex molecules in the oil to the shorter, simpler ones in the fuels.
Due to its high energy density, easy transportability and relative abundance, oil has become the world's most important source of energy since the mid-1950s. Petroleum is also the raw material for many chemical products, including pharmaceuticals, solvents,fertilizers, pesticides, and plastics; the 16 percent not used for energy production is converted into these other materials. Petroleum is found in porous rock formations in the upper strata of some areas of the Earth's crust. There is also petroleum in oil sands (tar sands). Known oil reserves are typically estimated at around 190 km3 (1.2 trillion (short scale) barrels) without oil sands,[44] or 595 km3 (3.74 trillion barrels) with oil sands.[45] Consumption is currently around 84 million barrels (13.4×106 m3) per day, or 4.9 km3 per year, yielding a remaining oil supply of only about 120 years, if current demand remains static.
Chemistry[edit]
Petroleum is a mixture of a very large number of different hydrocarbons; the most commonly found molecules are alkanes (paraffins), cycloalkanes (naphthenes), aromatic hydrocarbons, or more complicated chemicals likeasphaltenes. Each petroleum variety has a unique mix of molecules, which define its physical and chemical properties, like color and viscosity.
The alkanes, also known as paraffins, are saturated hydrocarbons with straight or branched chains which contain only carbon and hydrogen and have the general formula CnH2n+2 (where n is greater than 0). They generally have from 5 to 40 carbon atoms per molecule, although trace amounts of shorter or longer molecules may be present in the mixture.
The alkanes from pentane (C5H12) to octane (C8H18) are refined into gasoline, the ones from nonane (C9H20) to hexadecane (C16H34) into diesel fuel, kerosene and jet fuel. Alkanes with more than 16 carbon atoms can be refined into fuel oil and lubricating oil. At the heavier end of the range, paraffin wax is an alkane with approximately 25 carbon atoms, while asphalt has 35 and up, although these are usually cracked by modern refineries into more valuable products. The shortest molecules, those with four or fewer carbon atoms, are in a gaseous state at room temperature. They are the petroleum gases. Depending on demand and the cost of recovery, these gases are either flared off, sold as liquefied petroleum gas under pressure, or used to power the refinery's own burners. During the winter, butane (C4H10), is blended into the gasoline pool at high rates, because its high vapor pressure assists with cold starts. Liquified under pressure slightly above atmospheric, it is best known for powering cigarette lighters, but it is also a main fuel source for many developing countries. Propane can be liquified under modest pressure, and is consumed for just about every application relying on petroleum for energy, from cooking to heating to transportation.
The cycloalkanes, also known as naphthenes, are saturated hydrocarbons which have one or more carbon rings to which hydrogen atoms are attached according to the formula CnH2n. Cycloalkanes have similar properties to alkanes but have higher boiling points.
The aromatic hydrocarbons are unsaturated hydrocarbons which have one or more planar six-carbon rings called benzene rings, to which hydrogen atoms are attached with the formula CnHn. They tend to burn with a sooty flame, and many have a sweet aroma. Some are carcinogenic.
These different molecules are separated by fractional distillation at an oil refinery to produce gasoline, jet fuel, kerosene, and other hydrocarbons. For example, 2,2,4-trimethylpentane (isooctane), widely used in gasoline, has a chemical formula of C8H18 and it reacts with oxygen exothermically:[46]
- 2 C
8H18(l) + 25 O2(g) → 16 CO2(g) + 18 H2O(g) (ΔH = −5.51 MJ/mol of octane)
The number of various molecules in an oil sample can be determined in laboratory. The molecules are typically extracted in a solvent, then separated in a gas chromatograph, and finally determined with a suitable detector, such as a flame ionization detector or amass spectrometer.[47] Due to the large number of co-eluted hydrocarbons within oil, many cannot be resolved by traditional gas chromatography and typically appear as a hump in the chromatogram. This unresolved complex mixture (UCM) of hydrocarbons is particularly apparent when analysing weathered oils and extracts from tissues of organisms exposed to oil.
Incomplete combustion of petroleum or gasoline results in production of toxic byproducts. Too little oxygen results in carbon monoxide. Due to the high temperatures and high pressures involved, exhaust gases from gasoline combustion in car engines usually include nitrogen oxides which are responsible for creation of photochemical smog.
Empirical equations for thermal properties[edit]
Heat of combustion[edit]
At a constant volume the heat of combustion of a petroleum product can be approximated as follows:
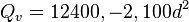 .
.
where  is measured in cal/gram and d is the specific gravity at 60 °F (16 °C).
is measured in cal/gram and d is the specific gravity at 60 °F (16 °C).
 is measured in cal/gram and d is the specific gravity at 60 °F (16 °C).
is measured in cal/gram and d is the specific gravity at 60 °F (16 °C).Thermal conductivity[edit]
The thermal conductivity of petroleum based liquids can be modeled as follows:[48]
where K is measured in BTU · °F−1hr−1ft−1 , t is measured in °F and API is degrees API gravity.
Specific heat[edit]
The specific heat of a petroleum oils can be modeled as follows:[49]
![c = \frac{1}{d} [0.388+0.00046t]](https://upload.wikimedia.org/math/f/f/2/ff214f4d61b089254f5f4d1312766372.png) ,
,
where c is measured in BTU/lbm-°F, t is the temperature in Fahrenheit and d is the specific gravity at 60 °F (16 °C).
In units of kcal/(kg·°C), the formula is:
![c = \frac{1}{d} [0.4024+0.00081t]](https://upload.wikimedia.org/math/6/0/4/604ad5ee4cf47000104a0683f00f698e.png) ,
,
where the temperature t is in Celsius and d is the specific gravity at 15 °C.
Latent heat of vaporization[edit]
The latent heat of vaporization can be modeled under atmospheric conditions as follows:
![L = \frac{1}{d}[110.9 - 0.09t]](https://upload.wikimedia.org/math/d/4/8/d48ce3476cf3f07d3ddfbd920957e966.png) ,
,
where L is measured in BTU/lbm, t is measured in °F and d is the specific gravity at 60 °F (16 °C).
In units of kcal/kg, the formula is:
![L = \frac{1}{d}[194.4 - 0.162t]](https://upload.wikimedia.org/math/a/a/a/aaa02f4901378661716a4658785bcdad.png) ,
,
where the temperature t is in Celsius and d is the specific gravity at 15 °C.[50]
Formation[edit]
Petroleum is a fossil fuel derived from ancient fossilized organic materials, such as zooplankton and algae.[53] Vast quantities of these remains settled to sea or lake bottoms, mixing with sediments and being buried under anoxic conditions. As further layers settled to the sea or lake bed, intense heat and pressure build up in the lower regions. This process caused the organic matter to change, first into a waxy material known as kerogen, which is found in various oil shales around the world, and then with more heat into liquid and gaseous hydrocarbons via a process known as catagenesis. Formation of petroleum occurs from hydrocarbon pyrolysis in a variety of mainly endothermic reactions at high temperature and/or pressure.[54]
There were certain warm nutrient-rich environments such as the Gulf of Mexico and the ancient Tethys Sea where the large amounts of organic material falling to the ocean floor exceeded the rate at which it could decompose. This resulted in large masses of organic material being buried under subsequent deposits such as shale formed from mud. This massive organic deposit later became heated and transformed under pressure into oil.[55]
Geologists often refer to the temperature range in which oil forms as an "oil window"[56]—below the minimum temperature oil remains trapped in the form of kerogen, and above the maximum temperature the oil is converted to natural gas through the process of thermal cracking. Sometimes, oil formed at extreme depths may migrate and become trapped at a much shallower level. The Athabasca Oil Sands are one example of this.
An alternative mechanism was proposed by Russian scientists in the mid-1850s, the hypothesis of abiogenic petroleum origin, but this is contradicted by geological and geochemical evidence.[57]
Abiogenic (formed by inorganic means) sources of oil have been found, but never in commercially profitable amounts. The controversy isn't over whether naturally forming oil reserves exist, said Larry Nation of the American Association of Petroleum Geologists. It's over how much they contribute to Earth's overall reserves and how much time and effort geologists should devote to seeking them out.[58]
Reservoirs[edit]
Crude oil reservoirs[edit]
Three conditions must be present for oil reservoirs to form: a source rock rich in hydrocarbon material buried deep enough for subterranean heat to cook it into oil, a porous and permeable reservoir rock for it to accumulate in, and a cap rock (seal) or other mechanism that prevents it from escaping to the surface. Within these reservoirs, fluids will typically organize themselves like a three-layer cake with a layer of water below the oil layer and a layer of gas above it, although the different layers vary in size between reservoirs. Because most hydrocarbons are less dense than rock or water, they often migrate upward through adjacent rock layers until either reaching the surface or becoming trapped within porous rocks (known as reservoirs) by impermeable rocks above. However, the process is influenced by underground water flows, causing oil to migrate hundreds of kilometres horizontally or even short distances downward before becoming trapped in a reservoir. When hydrocarbons are concentrated in a trap, an oil field forms, from which the liquid can be extracted by drilling and pumping.
The reactions that produce oil and natural gas are often modeled as first order breakdown reactions, where hydrocarbons are broken down to oil and natural gas by a set of parallel reactions, and oil eventually breaks down to natural gas by another set of reactions. The latter set is regularly used in petrochemical plants and oil refineries.
Wells are drilled into oil reservoirs to extract the crude oil. "Natural lift" production methods that rely on the natural reservoir pressure to force the oil to the surface are usually sufficient for a while after reservoirs are first tapped. In some reservoirs, such as in the Middle East, the natural pressure is sufficient over a long time. The natural pressure in most reservoirs, however, eventually dissipates. Then the oil must be extracted using "artificial lift" means. Over time, these "primary" methods become less effective and "secondary" production methods may be used. A common secondary method is "waterflood" or injection of water into the reservoir to increase pressure and force the oil to the drilled shaft or "wellbore." Eventually "tertiary" or "enhanced" oil recovery methods may be used to increase the oil's flow characteristics by injecting steam, carbon dioxide and other gases or chemicals into the reservoir. In the United States, primary production methods account for less than 40 percent of the oil produced on a daily basis, secondary methods account for about half, and tertiary recovery the remaining 10 percent. Extracting oil (or "bitumen") from oil/tar sand and oil shale deposits requires mining the sand or shale and heating it in a vessel or retort, or using "in-situ" methods of injecting heated liquids into the deposit and then pumping out the oil-saturated liquid.
Unconventional oil reservoirs[edit]
Oil-eating bacteria biodegrade oil that has escaped to the surface. Oil sands are reservoirs of partially biodegraded oil still in the process of escaping and being biodegraded, but they contain so much migrating oil that, although most of it has escaped, vast amounts are still present—more than can be found in conventional oil reservoirs. The lighter fractions of the crude oil are destroyed first, resulting in reservoirs containing an extremely heavy form of crude oil, called crude bitumen in Canada, or extra-heavy crude oil inVenezuela. These two countries have the world's largest deposits of oil sands.
On the other hand, oil shales are source rocks that have not been exposed to heat or pressure long enough to convert their trapped hydrocarbons into crude oil. Technically speaking, oil shales are not always shales and do not contain oil, but are fined-grain sedimentary rocks containing an insoluble organic solid called kerogen. The kerogen in the rock can be converted into crude oil using heat and pressure to simulate natural processes. The method has been known for centuries and was patented in 1694 under British Crown Patent No. 330 covering, "A way to extract and make great quantities of pitch, tar, and oil out of a sort of stone." Although oil shales are found in many countries, the United States has the world's largest deposits.[59]
Classification[edit]
See also: Benchmark (crude oil)
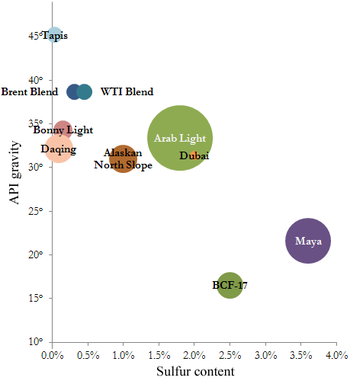
The petroleum industry generally classifies crude oil by the geographic location it is produced in (e.g. West Texas Intermediate, Brent, or Oman), its API gravity (an oil industry measure of density), and its sulfur content. Crude oil may be considered light if it has low density or heavy if it has high density; and it may be referred to as sweet if it contains relatively little sulfur or sour if it contains substantial amounts of sulfur.
The geographic location is important because it affects transportation costs to the refinery. Light crude oil is more desirable than heavy oil since it produces a higher yield of gasoline, while sweet oil commands a higher price than sour oil because it has fewer environmental problems and requires less refining to meet sulfur standards imposed on fuels in consuming countries. Each crude oil has unique molecular characteristics which are understood by the use of Crude oil assay analysis in petroleum laboratories.
Barrels from an area in which the crude oil's molecular characteristics have been determined and the oil has been classified are used as pricing references throughout the world. Some of the common reference crudes are:
- West Texas Intermediate (WTI), a very high-quality, sweet, light oil delivered at Cushing, Oklahoma for North American oil
- Brent Blend, comprising 15 oils from fields in the Brent and Ninian systems in the East Shetland Basin of the North Sea. The oil is landed at Sullom Voe terminal in Shetland. Oil production from Europe, Africa and Middle Eastern oil flowing West tends to be priced off this oil, which forms a benchmark
- Dubai-Oman, used as benchmark for Middle East sour crude oil flowing to the Asia-Pacific region
- Tapis (from Malaysia, used as a reference for light Far East oil)
- Minas (from Indonesia, used as a reference for heavy Far East oil)
- The OPEC Reference Basket, a weighted average of oil blends from various OPEC (The Organization of the Petroleum Exporting Countries) countries
- Midway Sunset Heavy, by which heavy oil in California is priced[60]
- Western Canadian Select the benchmark crude oil for emerging heavy, high TAN (acidic) crudes.[61]
There are declining amounts of these benchmark oils being produced each year, so other oils are more commonly what is actually delivered. While the reference price may be for West Texas Intermediate delivered at Cushing, the actual oil being traded may be a discounted Canadian heavy oil—Western Canadian Select— delivered at Hardisty, Alberta, and for a Brent Blend delivered at Shetland, it may be a Russian Export Blend delivered at the port of Primorsk.[62]
Petroleum industry[edit]
Main article: Petroleum industry
The petroleum industry is involved in the global processes of exploration, extraction, refining, transporting (often with oil tankers and pipelines), and marketing petroleum products. The largest volume products of the industry are fuel oil and gasoline. Petroleum is also the raw material for many chemical products, including pharmaceuticals, solvents, fertilizers, pesticides, and plastics. The industry is usually divided into three major components: upstream, midstream and downstream. Midstream operations are usually included in the downstream category.
Petroleum is vital to many industries, and is of importance to the maintenance of industrialized civilization itself, and thus is a critical concern to many nations. Oil accounts for a large percentage of the world's energy consumption, ranging from a low of 32 percent for Europe and Asia, up to a high of 53 percent for the Middle East, South and Central America (44%), Africa (41%), and North America (40%). The world at large consumes 30 billion barrels (4.8 km³) of oil per year, and the top oil consumers largely consist of developed nations. In fact, 24 percent of the oil consumed in 2004 went to the United States alone,[64] though by 2007 this had dropped to 21 percent of world oil consumed.[65]
In the US, in the states of Arizona, California, Hawaii, Nevada, Oregon and Washington, the Western States Petroleum Association (WSPA) represents companies responsible for producing, distributing, refining, transporting and marketing petroleum. This non-profit trade association was founded in 1907, and is the oldest petroleum trade association in the United States.[66]
Shipping[edit]
In the 1950s, shipping costs made up 33 percent of the price of oil transported from the Persian Gulf to USA,[67] but due to the development of supertankers in the 1970s, the cost of shipping dropped to only 5 percent of the price of Persian oil in USA.[67] Due to the increase of the value of the crude oil during the last 30 years, the share of the shipping cost on the final cost of the delivered commodity was less than 3% in 2010. For example, in 2010 the shipping cost from the Persian Gulf to the USA was in the range of 20 $/t and the cost of the delivered crude oil around 800 $/t.[citation needed]
Price[edit]
Main article: Price of oil
After the collapse of the OPEC-administered pricing system in 1985, and a short lived experiment with netback pricing, oil-exporting countries adopted a market-linked pricing mechanism.[68] First adopted by PEMEX in 1986, market-linked pricing was widely accepted, and by 1988 became and still is the main method for pricing crude oil in international trade.[68] The current reference, or pricing markers, are Brent, WTI, and Dubai/Oman.[68]







![K = \frac{1.62}{API}[1-0.0003(t-32)]](https://upload.wikimedia.org/math/2/5/0/25095c0e8a9c6432c80fde7cf4710844.png)